Awesome Info About How To Tell If Acid Is Strong Or Weak

How to find a method.
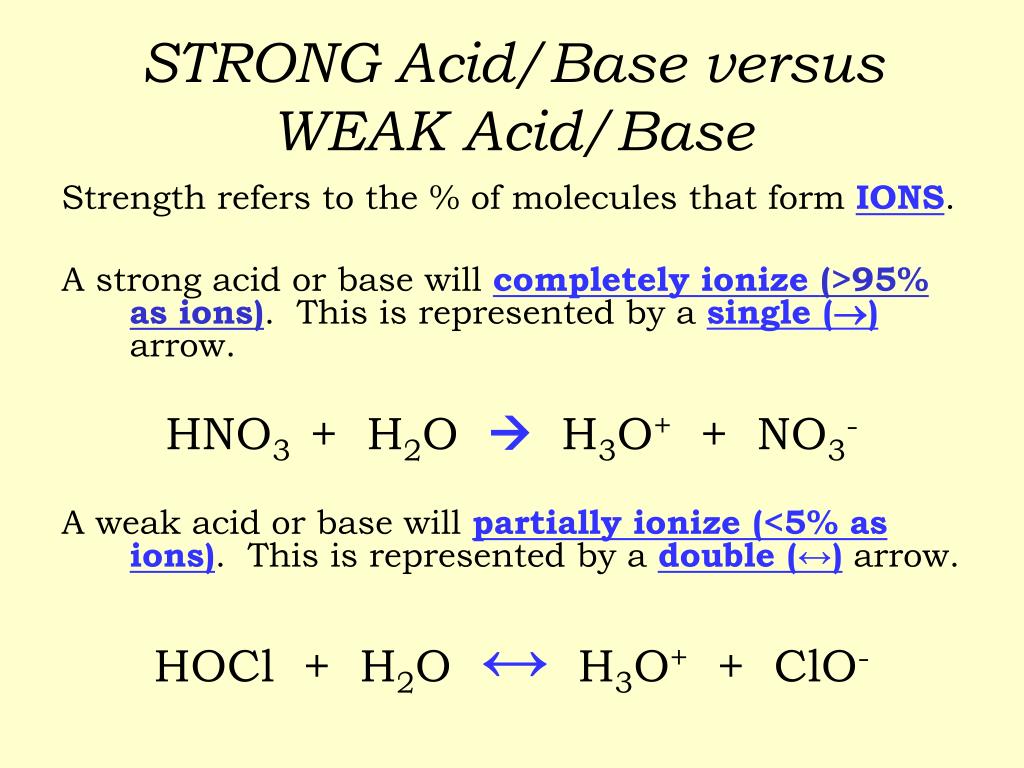
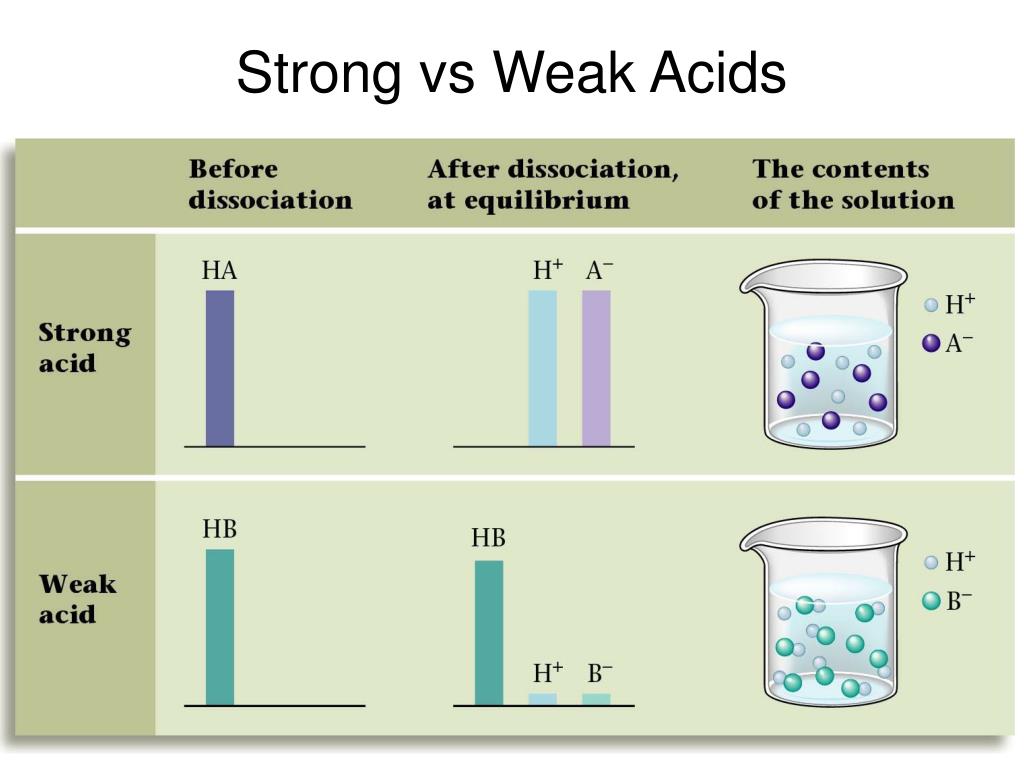
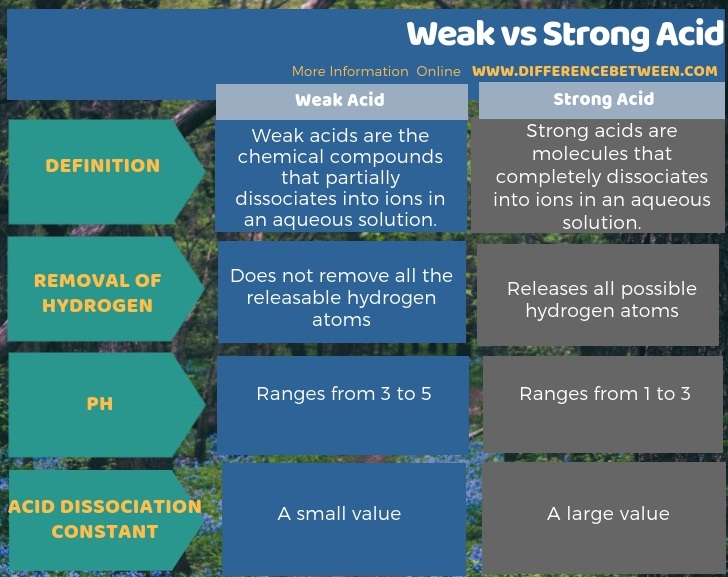
How to tell if acid is strong or weak. Result how can you tell if an acid or a base is strong or weak by looking at a sketch of its molecules? Result strong and weak acids. Ha (aq) + oh⁻ (aq) → a⁻ (aq) + h₂o (l).
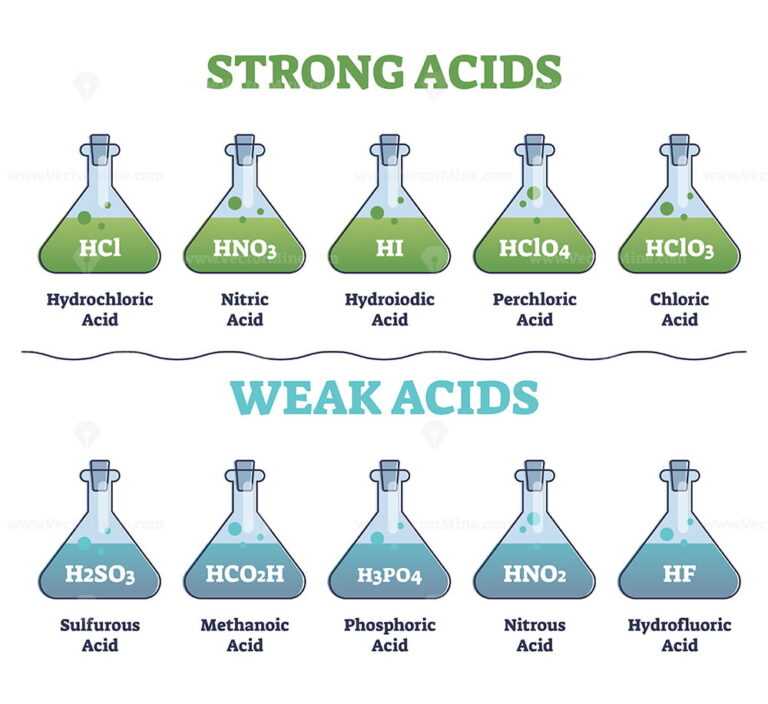
Examples of weak acids include hydrofluoric acid, hf, and acetic acid,. Result recognize an acid or a base as strong or weak. Except for their names and formulas, so far we have.
If an acid is not listed here, it is a weak acid. It may be 1% ionized or 99% ionized, but it is still classified as a weak acid. Determine if a salt produces an acidic or a basic solution.
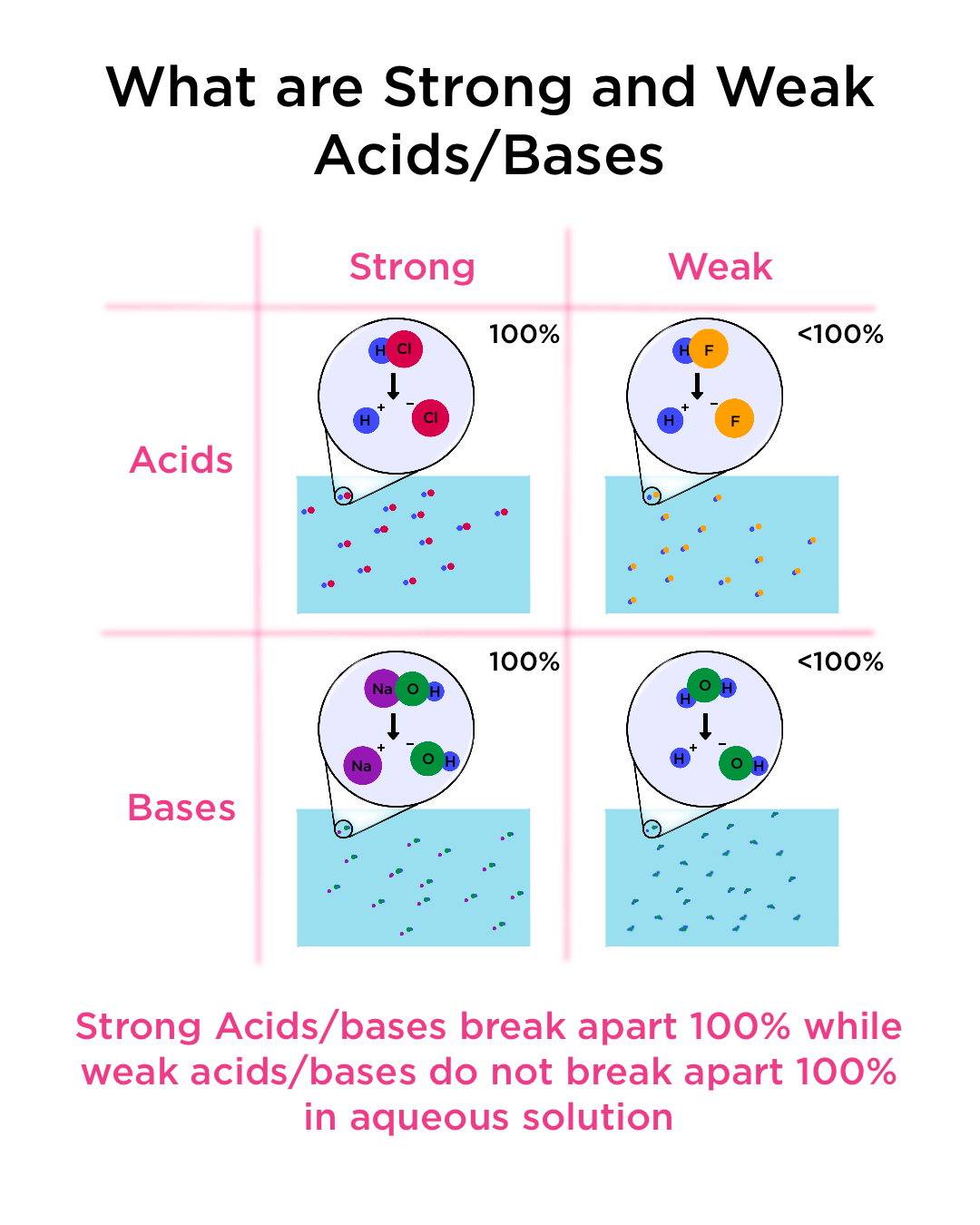
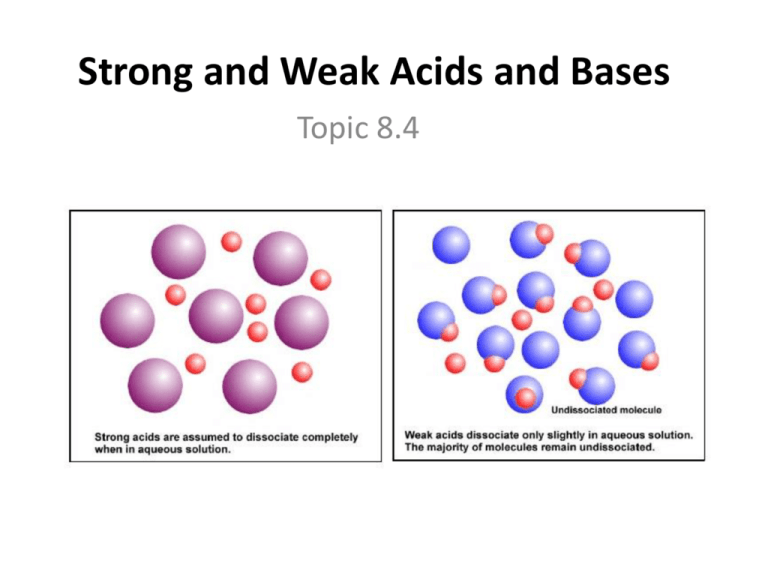
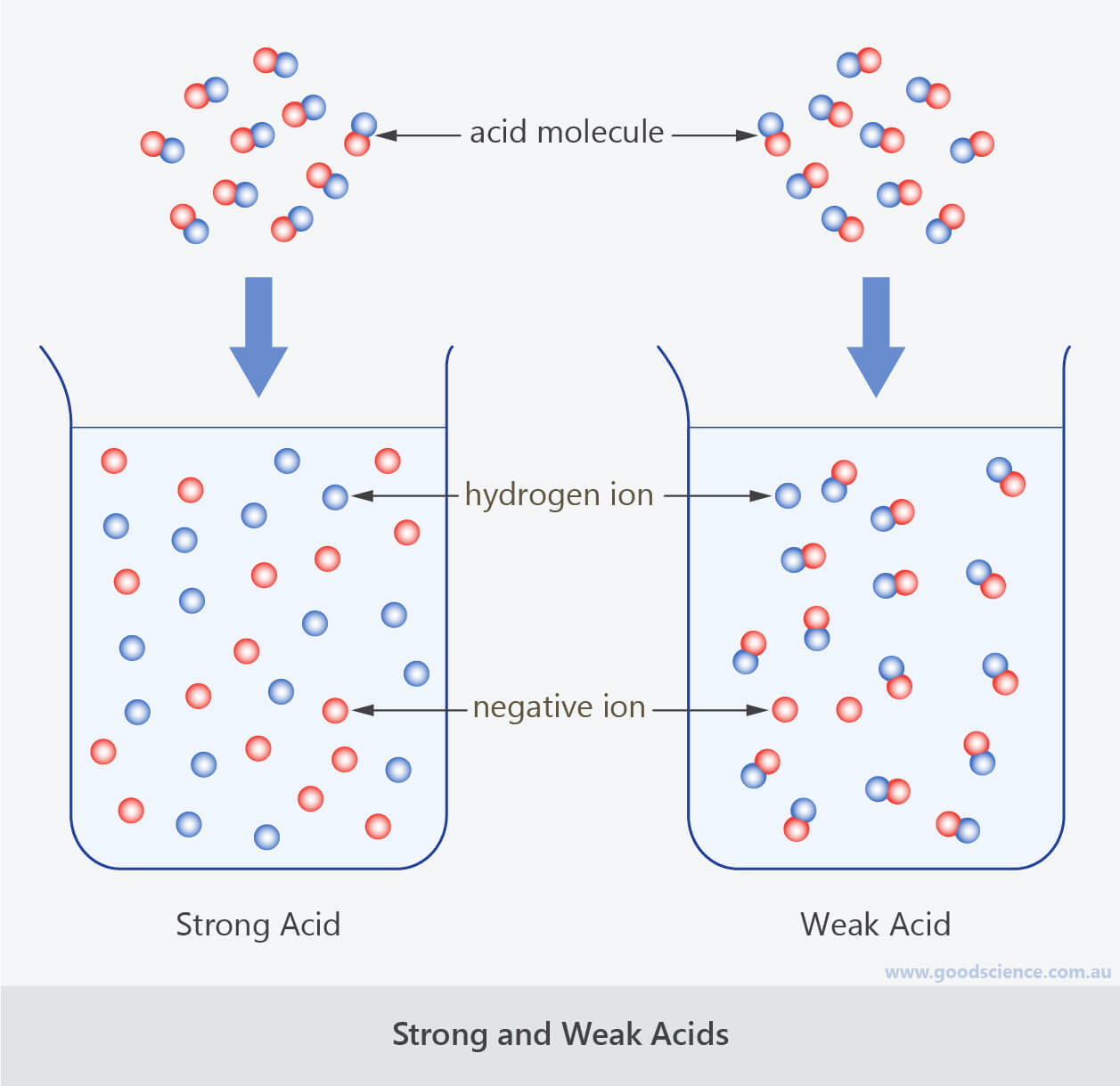
Watch this video to learn how to identify the key features. Identifying weak bases and strong bases. Strong acids dissociate fully in water to produce the maximum number of h + ions.
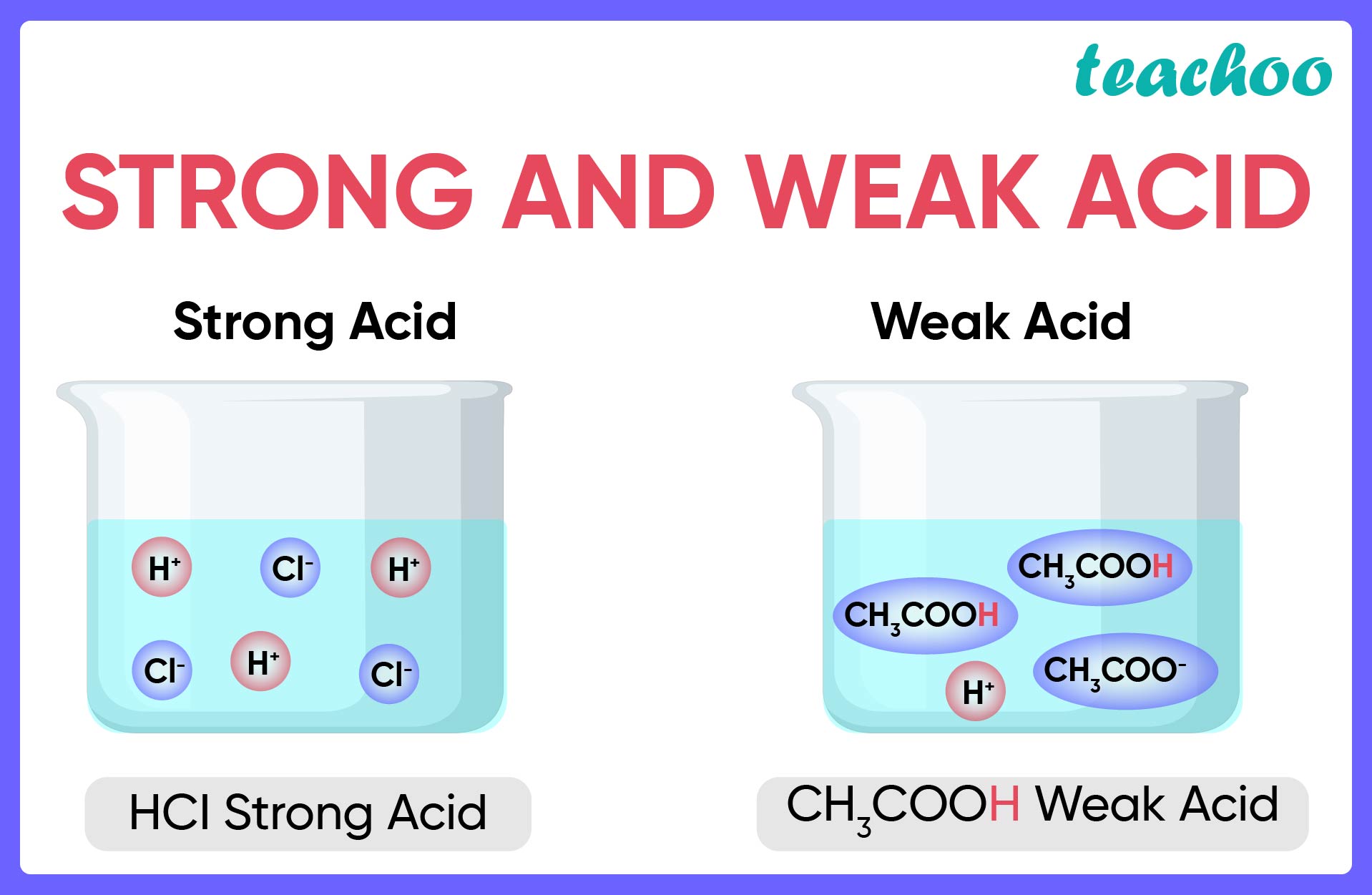
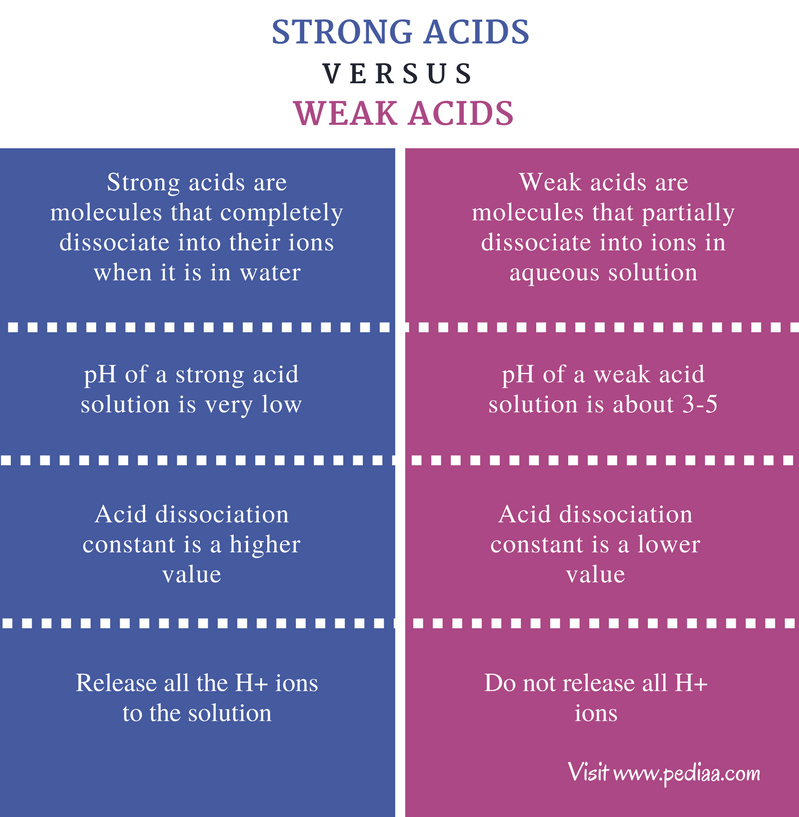
Result there are very few strong acids, so one of the easiest ways to tell strong and weak acids apart is to memorize the short list of strong ones. Result as it turns out, there are very few strong acids, which are given in table 14.7.1. Result the main difference between strong and weak acids is that strong acids dissociate completely in aqueous solutions whereas weak acids partially.
Result a weak acid only partially dissociates in water to give h + and the anion.

:max_bytes(150000):strip_icc()/list-of-strong-and-weak-acids-603642-v2copy2-5b47abd0c9e77c001a395e55.png)